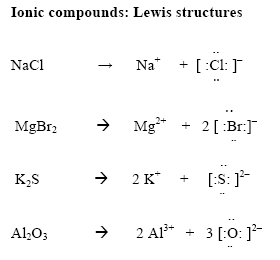First Class Tips About How To Draw Lewis Structures For Ions

Lewis structures for polyatomic ions.
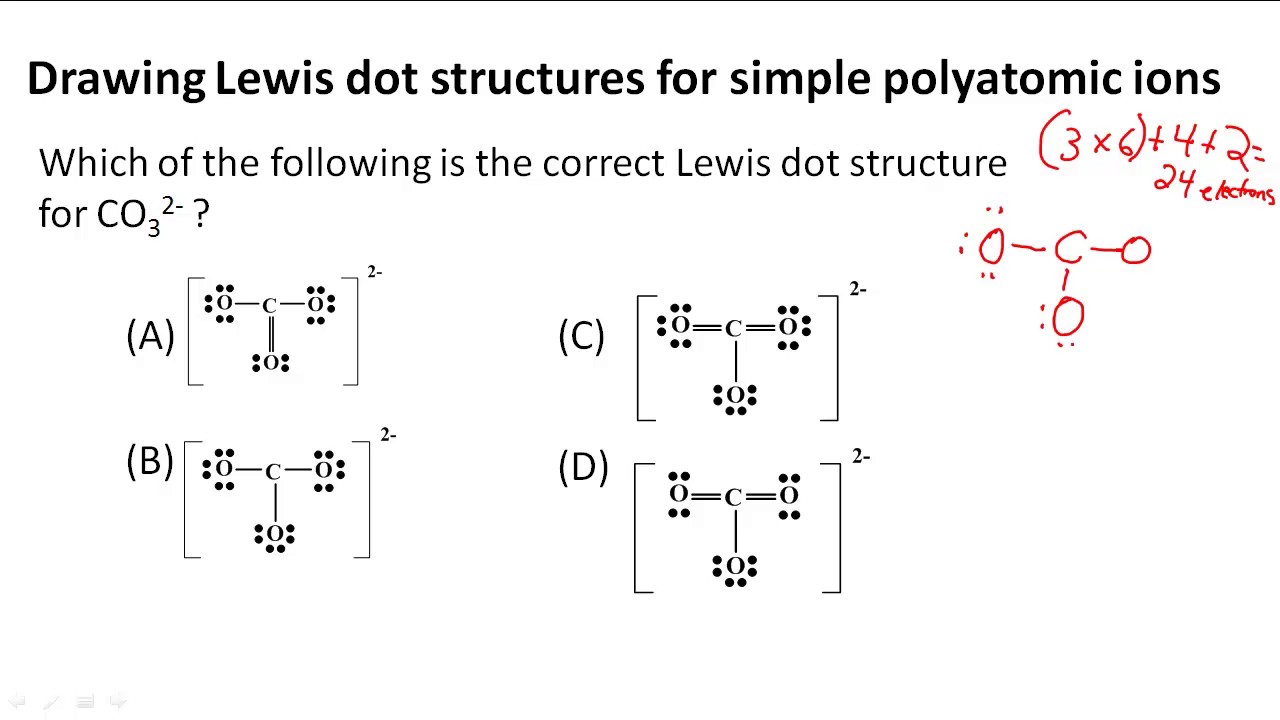
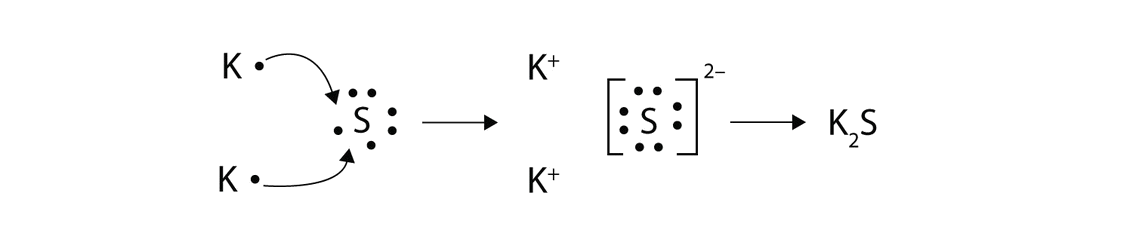
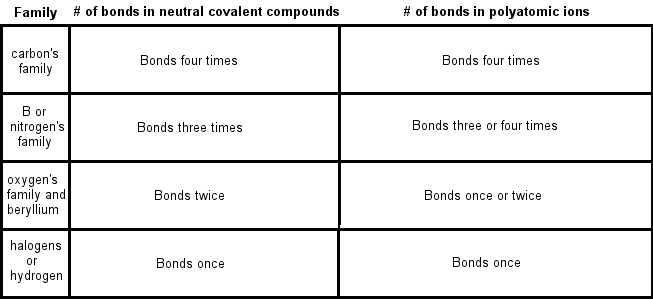
How to draw lewis structures for ions. Using the periodic table we. For ionic compounds with polyatomic ions, like nano3 or k2so4, we need to draw the lewis structure for the polyatomic (which is usually a covalent compound where valence. We first count the number of valence electrons for the polyatomic ion.
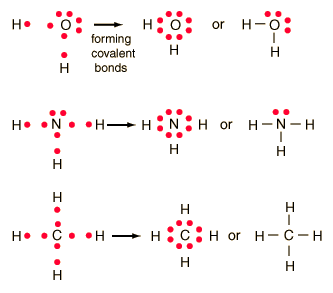
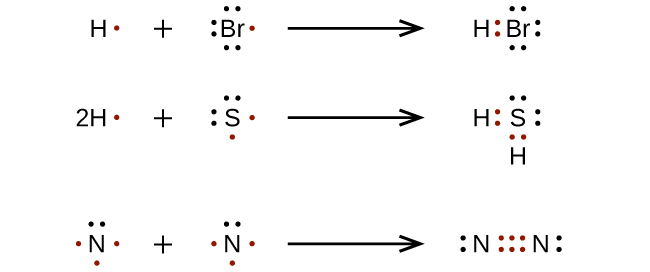
Examples include nacl, mgf2, k2o, and al2o3.my website: How to draw lewis structures. We will also go over what the octet rule is and all the e.
This organic chemistry video tutorial explains how to draw lewis structures using a simple method. Ad over 27,000 video lessons and other resources, you're guaranteed to find what you need. Some theories of aging suggest that free radicals cause certain diseases.
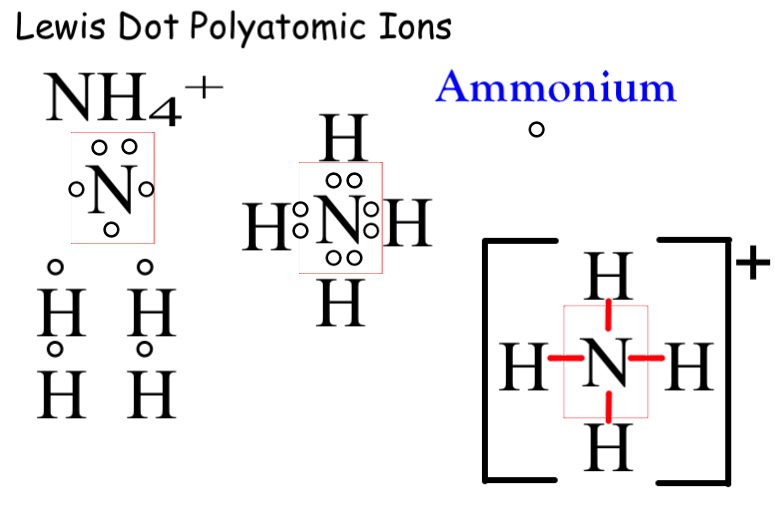
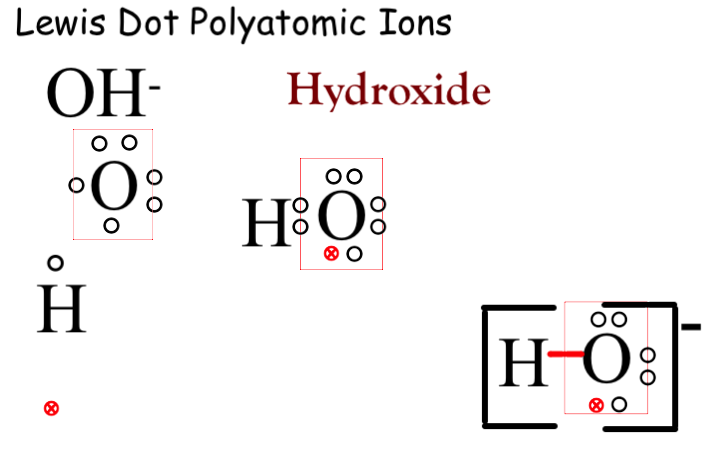
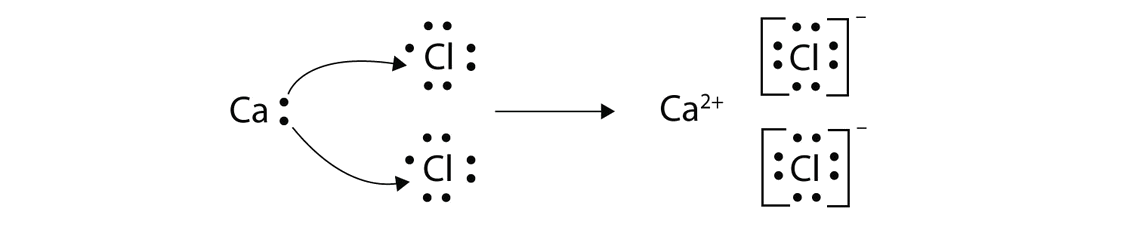
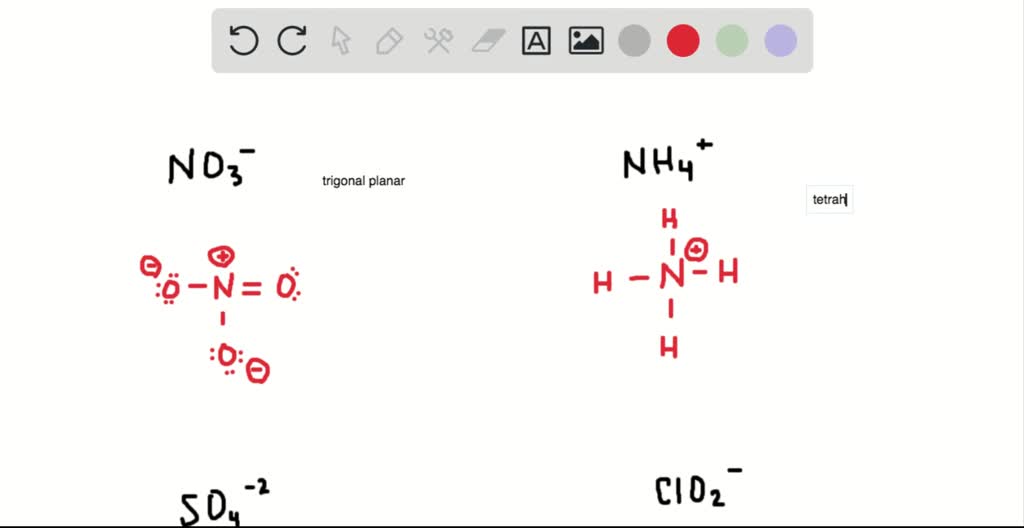
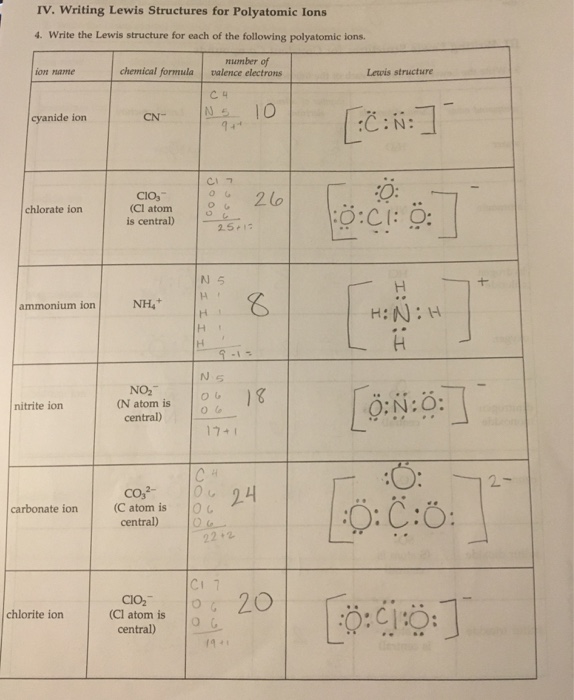
Find the number of valence electrons. This is done in the same way as for neutral ions, except that when you’re done, you need to change the number of electrons to. When the lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets.
However, since it is an ion, it would be beneficial to draw the. Making lewis structures for ions 1 write the atomic symbol. Remember that lewis dot structures only give reasonable results for covalent compounds.
How to draw lewis structures 1. I'll cover how to properly draw lewis structures of regular molecules and lewis structures of ions. Drawing lewis structures for ions.